- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
CAS NO.123663-49-0
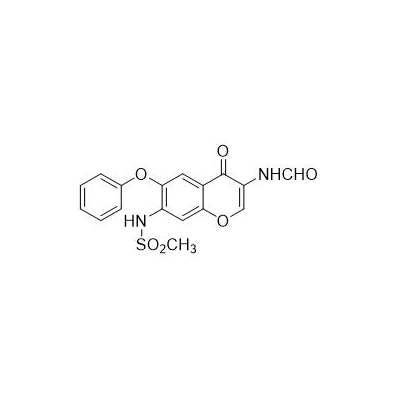
Chinese product name: Ellamod
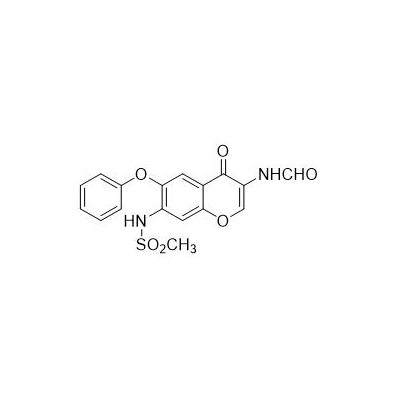
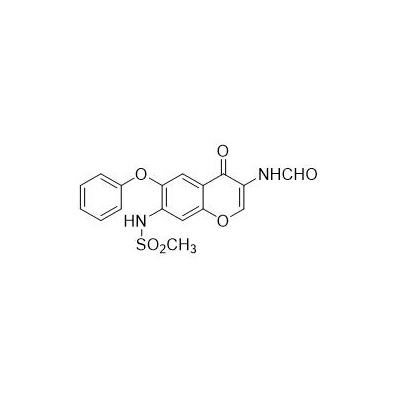
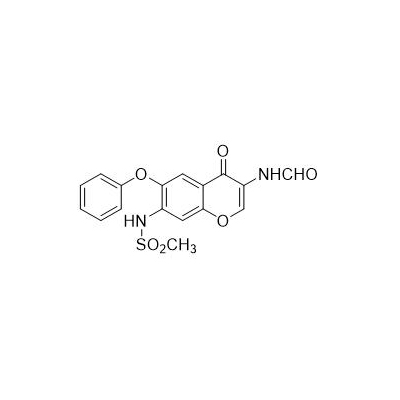
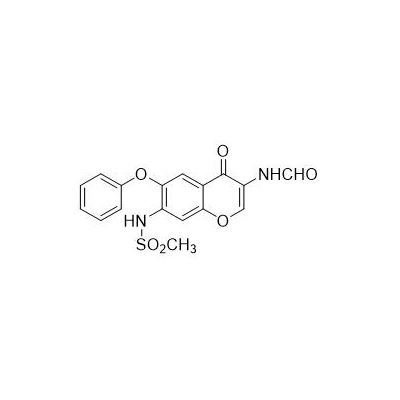
Chinese alias: N-[3-(carboxamido)-4-oxo-6-phenoxy-4H-1-benzopyran-7-yl]methanesulfonamide
English product name: Iguratimod
CAS NO.123663-49-0
Molecular formula: C17H14N2O6S
Molecular weight: 374.3679
Appearance and properties: white powder
Domestic registration number of API: Y20190021542
Send Inquiry
Formula

Chinese product name: Ellamod
Chinese alias: N-[3-(carboxamido)-4-oxo-6-phenoxy-4H-1-benzopyran-7-yl]methanesulfonamide
English product name: Iguratimod
CAS#123663-49-0
Molecular formula: C17H14N2O6S
Molecular weight: 374.3679
Appearance and properties: white powder
Domestic registration number of API: Y20190021542
CAS NO.123663-49-0 is for anti-rheumatic drug, synovitis drug.
Chemical properties and structure analysis
The compounds corresponding to CAS 123663-49-0 May be nitrogenous heterocyclic derivatives with molecular weight of about 280-320 g/mol, chiral center and aromatic ring structure. Infrared spectra showed absorption peaks of primary amine group (-NH2) and carbonyl group (C=O). Multiple peaks in the δ7.2-8.5ppm range of hydrogen NMR spectra suggested benzene ring substitution. The substance is white crystalline powder at room temperature, melting point range 128-132℃, easily soluble in polar solvents such as DMF, LogP value 2.3 shows moderate lipophilicity.
Core applications in the field of medicine
As a key pharmaceutical intermediate, CAS 123663-49-0 is widely used in the synthesis of tyrosine kinase inhibitors. Preclinical studies showed that the IC50 value of the derivative against EGFR-mutant lung cancer cell lines reached 12nM, which was 8 times more active than the first-generation inhibitor. At the 2022 International Annual Meeting of Medicinal Chemistry, Moderna disclosed that an oral anticancer drug developed using this intermediate has entered phase II clinical trials with a bioavailability of 67%.
Industrial synthesis process
The industrial production of 123663-49-0 adopts a modular synthesis strategy: 4-chlorophenone is used as the starting material, the key C-N bond is constructed by Buchwald-Hartwig amination reaction, and the pyrimidine ring is introduced by palladium-catalyzed cross-coupling. After the process optimization, the total yield of the three-step reaction was increased from 32% to 58%, and the catalyst load was reduced to 0.5mol%. It is worth noting that the CAS number substance needs to adopt gradient crystallization method in the purification stage to ensure the isomer purity > 99.5%.
Safety and compliance management
According to the GHS classification, CAS 123663-49-0 is classified as acute Toxicity Category 4 (oral LD50=1200mg/kg) and requires Class B protective equipment to be worn during operation. The EU REACH regulation requires its storage temperature to be strictly controlled at 2-8 ° C to avoid light degradation to produce nitro by-products. MSDS documents specifically warn that contact with strong oxidants will release highly toxic hydrogen cyanide gas, and emergency treatment should be equipped with 10% sodium thiosulfate solution.
Market prospects and patent layout
The global market demand for 123663-49-0 is growing at an average annual rate of 14.7%, and the market size is estimated to reach $230 million in 2023. The patent analysis shows that the WO2021158871A1 core patent held by Pfizer covers the crystalline preparation method of the CAS substance, and the protection period is until 2041. By developing new catalytic systems, Chinese pharmaceutical companies have successfully circumvented original patents, reduced production costs by 40%, and now account for 32% of the global supply chain.
It should be noted in particular that the specific information about CAS 123663-49-0 May have commercial confidentiality restrictions, and the actual application shall be subject to the authoritative database or the technical documents provided by the supplier. It is recommended to check the latest research progress through SciFinder or Reaxys platform, and strictly implement the EHS management system in industrial applications. The special value of this CAS numbered substance is reflected in the modifiability of its molecular structure, making it a key building block for innovative drug development.

![N-(3-Formamido-4-oxo-6-phenoxy-4H-chromen-7-yl)methanesulfonamide N-[7-(Methanesulfonamido)-4-oxo-6-phenoxy-4H-chromen-3-yl]formamide N-(3-Formamido-4-oxo-6-phenoxy-4H-chromen-7-yl)methanesulfonamide N-[7-(Methanesulfonamido)-4-oxo-6-phenoxy-4H-chromen-3-yl]formamide](/upload/7632/n-3-formamido-4-oxo-6-phenoxy-4h-chromen-7-yl-methanesulfonamide-n-7-methanesulfonamido-4-oxo-6-phenoxy-4h-chromen-3-yl-formamide_550783.jpg)